Soluble Salts List
Sodium chloride and potassium chloride. What are the insoluble salts.

List Of Some Poorly Soluble Drugs Their Salt Forms And Degree Of Download Scientific Diagram
Many specifiers fail to specify low allowable concentrations of soluble salts for fear of cost.
. We can use the K sp value for the salt to calculate the molar solubility. Some Li are insoluble with Li 3. Halides salts of Cl Br and I.
Shop Soluble Salt Testers at Grainger. What are the soluble salts. Per 100 ml of water at 0ÂC.
Essentially all alkali metal Li Na K Rb Cs and ammonium NH 4 salts are soluble. Ad Americas Trusted Source for Industrial Supplies with over 1. In house plants signs of excess soluble salts include reduced growth brown leaf tips dropping of lower leaves.
Soluble salts as referred to in soil science are those inorganic chemicals that are more soluble than gypsum CaSO 4 2H 2 O which has a solubility of 0241 gm. The salts in this group are very soluble in water because they have large ions and. Oxalates salts of C 2O2 4.
Solutions should be the cations of cupric nitrate ammonium nitrate zinc nitrate. Sodium Na Potassium K Chloride Cl- Calcium Ca2 Magnesium Mg2 B. The table below provides information on the variation of solubility of different substances mostly inorganic compounds in water with temperature at one atmosphere pressureUnits of solubility are given in grams per 100 millilitres of water g100 mL unless shown otherwise.
Salts may also build up on the outside of clay pots. Largest Distributor in the US. The substances are listed in alphabetical order.
For a full list of topics. The general rules for predicting the solubility of salts in water. 3051 135Aluminum alkyls Formula.
Most salts dissolve but not all. Answer 1 of 3. Chromates salts of CrO2 4.
Scaling occurs when sparingly soluble salts get concentrated beyond their solubility limits in the reject stream of the membrane element. A The only slightly soluble salt that can be formed when these two solutions are mixed is BaSO 4 because NaCl is highly soluble. The Table of solubility product is given as Salt Ksp in the Handbook Section.
Common table salt NaCl has solubility nearly 150 times greater than gypsum 357 gm. Advanced Search Advanced Search The National Institute for Occupational Safety and Health NIOSH Section Navigation. By the way all nitrates are soluble in water so lead.
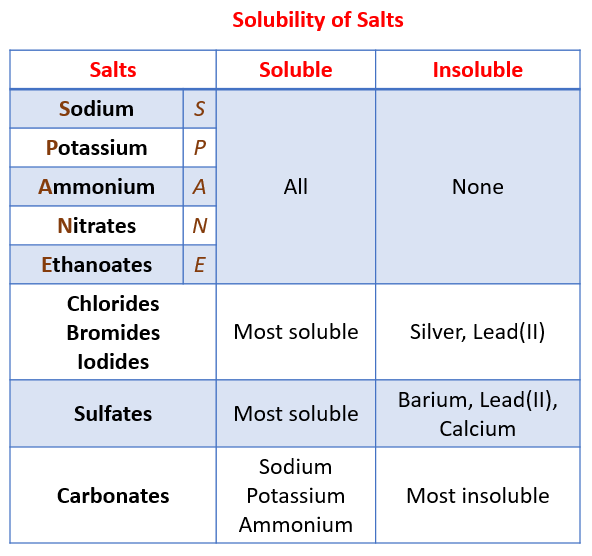
Calculate the molar solubility of AgCl in water. In this table the salts are divided into. Common Chlorides are soluble except Silver Chloride 4.
Sulfates salts of SO2 4. Synonyms Trade Names CAS No. Hydroxides salts of OH.
A ring of salt deposits may form around the pot at the soil line or around the drainage hole. Nature of Soluble Salts. DOT ID Guide.
Since salts are a relatively new subject to many people it is easy to understand that people are also not aware of the costs or methods of salt removal. Others simply do not want to spend any additional funds. All common Sodium Potassium and Ammonium salts are soluble 2.
The electrolytes that can be found in the body are the following. The equation for the precipitation of BaSO 4 is as follows. Carbonates salts of CO2 3.
The general table salt. -9-3-----33-21-K sp for Some Salts from Radel Navidi 2nd Ed Chromates Ag 2CrO 4 12x10. During the scaling process colloids of insoluble mineral salts are formed.
In this list you can easily notice the wide range in solubilities where silver nitrate has a very high solubility of 217 g per 100 g water and silver chloride has a very low solubility of 0000 000 1 g per 100 g water. Others include Ammonium nitrate and calcium nitrate. Then calculate the molar solubility of Ag 3PO 4 in water.
All Nitrates are soluble 3. Silver nitrite and potassium perchlorate are considered slightly soluble. BaSO4 s Ba2 aq SO2 4 aq The solubility product expression is as follows.
Ksp Ba 2 SO 42 10810 10. Columbia University in the City of New York. How to easily remember the soluble and insoluble saltsALSO WATCHCation Test - httpsyoutubeJJ.
Soluble salts may accumulate on the top of the soil forming a yellow or white crust. For sparingly soluble salts the value of K sp is quite small. All nitrate NO 3 nitrite NO 2 chlorate ClO 3 and perchlorate ClO 4 salts are soluble.
Minerals that precipitate and form scale are predominantly divalent metal ions such as calcium iron magnesium barium and silicon because they are almost insoluble in the. Aluminum soluble salts and alkyls as Al Related Pages. In this post the solubilities of various salts in water at 20 C are listed in a table figure 1.
Salts In Chemistry Preparation Types Properties And Uses Psiberg
Acid Bases Salts Igcse Chemistry Solutions Examples Worksheets Videos
What Are Some Soluble Salt Examples Quora

Difference Between Soluble And Insoluble Salts Compare The Difference Between Similar Terms
Comments
Post a Comment